 Until recently, urban ecologists focused mainly on patterns of species abundance and diversity. Only in recent years has research progressed into mechanistic urban ecology, with studies (mostly experimental) on behavioral ecology, species interactions, genetics, and evolution. Although this mechanistic approach is still in its infancy, it already indicates that the urban environment is a unique setting, in which fundamental patterns and processes can be decoupled by human activities. Thus, ecological “rules” in urban environments might differ dramatically from those in less human-influenced habitats. The tools used to understand these differences must also address the role of human behavior as a primary driving force of environmental change (Fig 1).
Until recently, urban ecologists focused mainly on patterns of species abundance and diversity. Only in recent years has research progressed into mechanistic urban ecology, with studies (mostly experimental) on behavioral ecology, species interactions, genetics, and evolution. Although this mechanistic approach is still in its infancy, it already indicates that the urban environment is a unique setting, in which fundamental patterns and processes can be decoupled by human activities. Thus, ecological “rules” in urban environments might differ dramatically from those in less human-influenced habitats. The tools used to understand these differences must also address the role of human behavior as a primary driving force of environmental change (Fig 1).
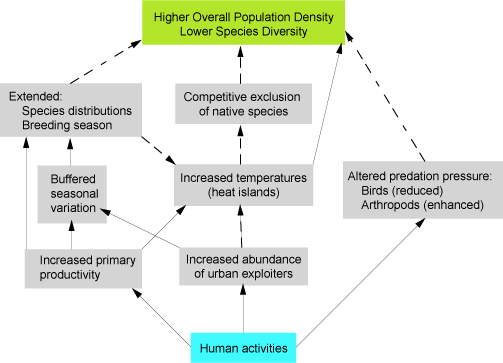
Animal diversity and abundance are altered radically in urban ecosystems relative to wildlands. Most species that thrive in urban environments are human-commensal species, some of which are synanthropic generalists. Others are less tolerant and could be on a slow march towards local extinction. Thus, although high resource availability supports high animal density in cities, local (alpha) diversity (i.e. within-patch diversity) tends to decline with increasing urbanization. These patterns indicate that, in contrast to wildlands, the number of individuals in urban areas is a poor predictor of species richness. Here, we highlight recent findings in mechanistic urban ecology that implicate several human-mediated processes in the production of urban patterns:
Habitat Productivity
Several studies describe a hump-shaped relationship for species richness versus level of urbanization. If net primary productivity is highest in moderately urbanized areas owing to the combination of low-density development and high anthropogenic resource inputs in managed green spaces, these studies are likely to reflect the signature of a hump-shaped species richness–productivity relationship (SRPR) along urbanization gradients. However, effects of urbanization on productivity can be diverse, depending on the ecological region and the growth form of a city. Thus, we hypothesize that, in managed green urban spaces, habitat productivity generally increases compared with surrounding wildlands. The productivity–diversity relationship is, however, expected to vary across different regions and in cities with different growth forms.
Species Interactions
One possible mechanism causing decreases in diversity as productivity increases is competition. Therefore, in addition to habitat loss, some species might fail to occupy urban habitats owing to increased competitive exclusion associated with the increase in habitat productivity. Understanding the key factors that should be controlled to reduce the density of urban exploiters might enable native species to tolerate urban conditions.
Thermal Habitat
The heat island effect can buffer against cold winters and extend growing seasons in temperate-zone cities, while adding thermal and drought stress in tropical, desert and subtropical cities; conversely, irrigated green spaces can buffer against high temperatures via evapotranspiration. We suggest that, in terms of contrast between hot–cold or rainy–dry seasons, cities can be viewed as “pseudo-tropical bubbles” regardless of their latitude, i.e., birds (e.g., European Magpie, Florida Scrub Jay) extend their breeding season because seasonal contrast is weaker.
Resource Availability
Urban management strategies often make spatially and temporally patchy resources more continuously available. Temporal changes in habitat structure and the availability of food and water are dampened in several cities.
Trophic Dynamics: Top-Down Versus Bottom-Up Control
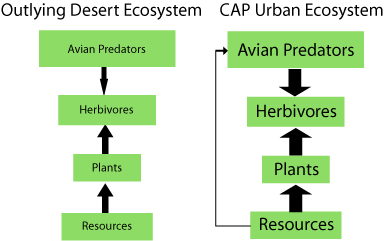
Control of food-web structure in urban communities is likely to differ greatly from that in wildlands. Recent experimental research in Phoenix, Arizona, finds that classic resource-based (bottom-up) and consumer-based (top-down) factors operate in urban communities, but their relative importance is altered compared with surrounding wildlands. The control of arthropod abundance shifts from almost exclusively bottom up in Sonoran Desert habitats to a combination of bottom-up and top-down forces in Phoenix (Fig. 2).
Evolution and Adaptation
Natural selection is short-circuited in many circumstances in urban environments. Selective pressures, such as temporal variation in food, water and predation, are often relaxed. Simultaneously, the novel environments constructed by humans in cities set new selective forces in motion, altering the behavior, morphology and genetic structure of populations. Behavioral flexibility might facilitate adaptation to these novel environments by some species.
The changes in ecological processes illustrated in the figure above should alter selective forces in cities, leading to the genetic differentiation of urban and wildlands populations or genetic changes associated with the fragmentation and isolation of wild populations. Alternatively, continuous migration to and from wildland habitats, as well as anthropogenic activities that construct and deconstruct entire biological communities, might prevent the genetic differentiation of urban populations and dampen evolutionary responses to these novel selective forces.
References
Baker, L.A., A. J. Brazel, N. Selover, C. Martin, N. McIntyre, F. R. Steiner, A. Nelson, and L. Musacchio. 2002. Urbanization and warming of Phoenix (Arizona, USA): Impacts, feedbacks, and mitigation. Urban Ecosystems 6(2002):183-203.
Brazel, A. J., N. Selover, R. Vose, and G. Heisler. 2000. The tale of two climates: Baltimore and Phoenix urban LTER sites. Climate Research 15(2):123-135.
Faeth, S. H., P. S. Warren, E. Shochat, and W. Marussich. 2005. Trophic dynamics in urban communities. BioScience 55(5):399-407.
McIntyre, N. E. 2000. Ecology of urban arthropods: A review and a call to action. Annals of the Entomological Society of America 93(4):825-835.
Shochat, E. 2004. Credit or debit? Resource input changes population dynamics of city-slicker birds. Oikos 106:622–626.
Shochat E., S. Lerman, M. Katti, and D. Lewis. 2004. Linking optimal foraging behavior to bird community structure in an urban-desert landscape: Field experiments with artificial food patches. American Naturalist 164(2):232-243.
Shochat, E., W. L. Stefanov, M. E. A. Whitehorse, and S. Faeth. 2004. Urbanization and spider diversity: Influences of human modification of habitat structure and productivity. Ecological Applications 14(4):268-280.
Shochat, E., P.S. Warren, S.H. Faeth, N.E. McIntyre, and D. Hope 2006. From patterns to emerging processes in mechanistic urban ecology. Trends in Ecology and Evolution 21:186-191.


